Chemistry in NEET isn’t just about memorizing facts; it’s about understanding each element and its unique behaviors. Each year, approximately 15-20% of NEET chemistry questions directly test your knowledge of elemental properties and their applications. From the smallest cation to the densest solid, each element’s characteristics can help students recall essential facts quickly and apply them during exams. Below, we explore twenty key properties and their corresponding elements, aiming to strengthen your knowledge for the NEET exam. Each property discussed here has been carefully selected based on its significance in previous NEET papers and its importance in chemistry.
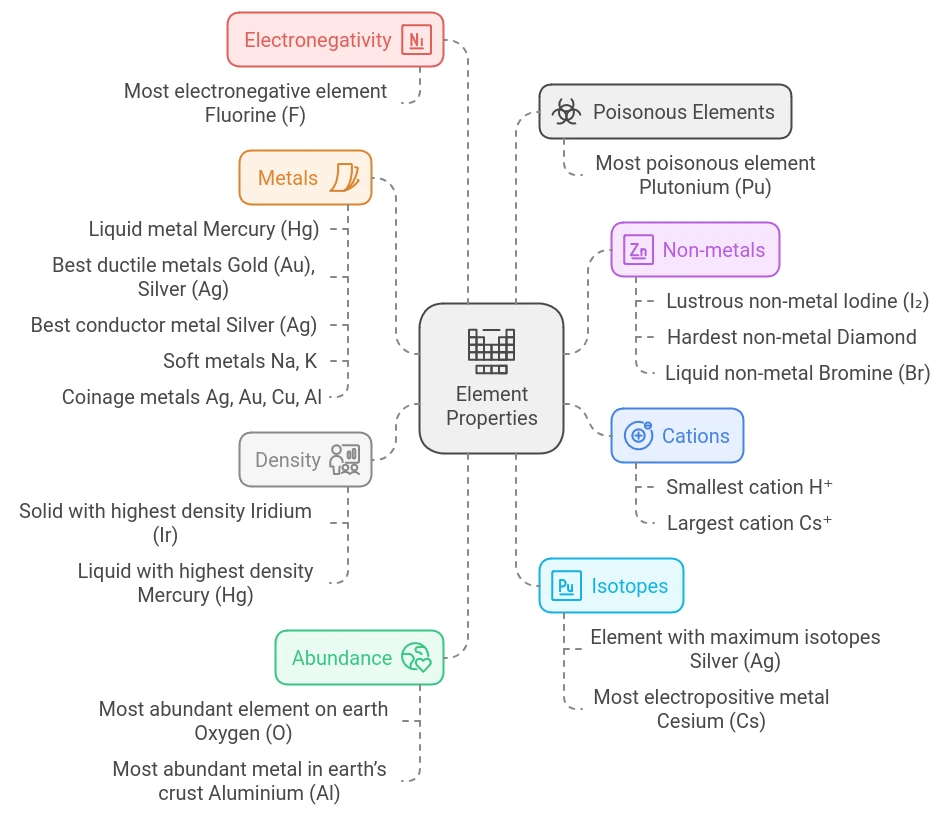
Top 20 Properties of Elements for NEET Chemistry
1. Smallest Cation: Hydrogen Ion (H⁺)
The hydrogen ion, represented as H⁺, holds the distinction of being the smallest cation in the periodic table. This is due to hydrogen’s unique atomic structure, which consists of only one proton and one electron. When hydrogen loses its single electron, it forms a positively charged ion, H⁺, which is essentially just a proton. This minimal size allows H⁺ to participate in various chemical reactions with ease, particularly in acid-base chemistry. In fact, the presence and concentration of hydrogen ions are what determine the acidity of a solution, making H⁺ fundamental to pH calculations and acid-base reactions, an important topic in NEET chemistry.
2. Largest Cation: Cesium Ion (Cs⁺)
Cesium (Cs) forms the largest cation, Cs⁺, which results from the loss of a single electron. Due to cesium’s large atomic radius and low ionization energy, it readily loses one electron to achieve the noble gas configuration of xenon. As an alkali metal in Group 1 of the periodic table, cesium is highly electropositive, meaning it easily forms cations by giving up its outermost electron. The large size of the Cs⁺ ion, coupled with its low density, makes cesium ideal for applications in atomic clocks and other precision instruments. This property also highlights the periodic trend of increasing atomic and ionic size as one moves down a group in the periodic table-a concept NEET aspirants should be familiar with.
3. Solid with Highest Density: Iridium (Ir)
Among the elements, iridium (Ir) is known as the densest naturally occurring solid, with a density of approximately 22.56 g/cm³. Iridium’s dense atomic packing and high atomic mass contribute to its exceptional density. This precious metal is part of the platinum group and is highly valued for its resistance to corrosion, even at high temperatures. Due to these properties, iridium finds applications in high-stress environments, including aerospace components, deep-sea equipment, and electrical contacts. Its density and durability also make it suitable for use in surgical tools and scientific apparatus. For NEET aspirants, understanding the correlation between atomic structure and density can be particularly useful in solving questions about physical properties of elements.
4. Liquid with Highest Density: Mercury (Hg)
Among liquids, mercury stands out as the densest, with a density of 13.53 g/cm³. Known as quicksilver, mercury is unique for being the only metal that is liquid under standard conditions.
5. Elements Named After Countries: Ru, Ge, Po, Am
Certain elements are named in honor of the countries associated with their discovery or significance. For example:
- Ruthenium (Ru) – Named after Russia
- Germanium (Ge) – Named after Germany
- Polonium (Po) – Named after Poland
- Americium (Am) – Named after America
These elements not only reflect the contributions of different countries to scientific advancement but also serve as examples of how the naming of elements commemorates cultural and geographical significance. NEET aspirants should be aware of such historical aspects, as they occasionally appear in exam questions on the origins of element names and properties of elements.
6. Most Electronegative Element: Fluorine (F)
Fluorine is the most electronegative element, with an electronegativity value of 3.98 on the Pauling scale. This extreme electronegativity means that fluorine has a very strong tendency to attract electrons from other elements, making it highly reactive, particularly with metals. Due to its reactivity, we commonly find fluorine in compounds rather than in its elemental form. It forms strong ionic bonds, as seen in compounds like sodium fluoride (NaF), which people widely use in dental health and industrial applications. Fluorine’s high electronegativity also explains its role in polar bonds and acid strength, important concepts in NEET chemistry.
7. Most Abundant Element on Earth: Oxygen (O)
Oxygen is the most abundant element on Earth, making up nearly 46% of the Earth’s crust and approximately 21% of the atmosphere by volume. This non-metal is essential for life, as it is involved in respiration, combustion, and oxidation processes. Oxygen exists in various forms, including O₂, which is essential for breathing, and O₃ (ozone), which protects us from harmful UV radiation in the atmosphere. Oxygen also combines with elements in rocks, minerals, and water, making it a central element in geological and biological processes. NEET aspirants should remember oxygen’s role in biological processes and its prevalence in Earth’s crust.
8. Most Abundant Metal in Earth’s Crust: Aluminium (Al)
Aluminium is the most abundant metal, constituting about 8.23% of the Earth’s crust. Its abundance, low density, and resistance to corrosion make aluminium a highly valuable metal in construction, transportation, and packaging. Due to its relatively reactive nature, aluminium is usually found in mineral compounds like bauxite rather than in its pure form. The production and recycling of aluminium are significant in industry, and its lightweight nature makes it essential in aerospace and automobile manufacturing. For NEET chemistry, understanding the extraction process of aluminium (the Bayer and Hall-Héroult processes) is also important.
9. Liquid Metal: Mercury (Hg)
Mercury, also known as quicksilver, is a unique metal that remains liquid at room temperature. Its unusual properties make it valuable in devices like thermometers and switches. Mercury’s high coefficient of thermal expansion allows it to respond to temperature changes with precision, which is why it has long been used in thermometers. However, mercury is toxic, and safer alternatives are now used in many applications. NEET aspirants should remember mercury’s chemical symbol (Hg) and its position as a transition metal in the periodic table.
10. Lustrous Non-metal: Iodine (I₂)
Iodine is one of the few non-metals that exhibits a metallic luster, displaying a shiny, violet-black appearance in its solid form. This lustrous quality, coupled with its sublimation property (transitioning directly from solid to gas), makes iodine unique among non-metals. Iodine is essential in human health, primarily used in thyroid hormone production, and iodine deficiency is a common issue worldwide. Additionally, iodine’s antiseptic properties make it valuable in medicine. NEET aspirants should remember iodine’s role in biochemistry, properties and its place in the halogen group elements.
11. Hardest Non-metal: Diamond
Diamond, an allotrope of carbon, is recognized as the hardest naturally occurring substance on Earth. Its hardness results from its tetrahedral crystal structure, where each carbon atom bonds covalently to four others, forming a rigid three-dimensional network. People prize diamonds in both industrial and ornamental applications, ranging from cutting tools to jewelry. In industry, people use diamonds to cut and drill hard materials, thanks to their unmatched durability. For NEET, it is important to note that diamond is an allotrope of carbon, and its hardness contrasts with other forms of carbon, such as graphite, which is soft and used as a lubricant.
12. Soft Metals: Sodium (Na) and Potassium (K)
Sodium and potassium are both alkali metals with a soft, malleable texture, making them easy to cut with a knife. Due to their highly reactive nature, especially with water, these metals can produce intense reactions, sometimes even igniting on contact. To prevent such dangerous reactions and to protect them from oxidation, we carefully store sodium and potassium under oil.
13. Best Ductile Metals: Gold (Au) and Silver (Ag)
Gold and silver are renowned for their exceptional ductility, meaning they can be drawn into extremely thin wires without breaking. This quality is due to their malleable atomic structure, which allows the atoms to slide past each other without fracturing. Gold, in particular, can be drawn into wires as thin as a single atom in diameter, making it invaluable in applications requiring thin, conductive wiring, such as microelectronics. Due to their resistance to tarnish and oxidation, gold and silver are also highly sought after in jewelry, where they retain their luster and beauty over time. NEET aspirants should recognize the practical importance of ductility in various industrial and technological contexts.
14. Best Conductor of Electricity: Silver (Ag)
Silver holds the title of the best conductor of electricity, thanks to its atomic structure, which allows electrons to flow with minimal resistance. This high conductivity is essential in applications that demand precision, such as in high-quality electrical contacts, circuits, and solar panels. Despite its superior conductivity, the high cost of silver limits its widespread use in electrical wiring, where copper often serves as a more affordable alternative. However, silver’s use remains vital in high-end electronics, medical devices, and specialized equipment, where maximum conductivity is critical. For NEET, it’s important to remember that conductivity depends on the material’s structure and that silver’s high conductivity can serve as a benchmark.
15. Most Poisonous Element: Plutonium (Pu)
Plutonium is notorious for being one of the most toxic and dangerous elements, mainly due to its radioactive properties. As an actinide, plutonium has a long half-life, and exposure to it can result in severe health risks, including radiation sickness and increased cancer risk. Despite its dangers, plutonium plays a role in nuclear reactors, where its radioactive decay generates energy. It is also a critical component in nuclear weapons. Handling plutonium requires stringent safety measures, as even trace amounts can contaminate the environment and harm human health. NEET students should understand both the toxicological and energy-producing aspects of radioactive elements like plutonium.
16. Element with Maximum Isotopes: Silver (Ag)
Silver is unique among elements for having a high number of isotopes—over 35 identified, with two stable isotopes, Ag-107 and Ag-109. Isotopes are atoms of the same element with different numbers of neutrons, and silver’s extensive range of isotopes offers valuable information about atomic behavior. The isotopic variations of silver are studied in nuclear physics and have applications in dating geological and archaeological samples. Understanding isotopes is crucial for NEET aspirants, as they play significant roles in various scientific fields, including chemistry, physics, and environmental science.
17. Most Electropositive Metal: Cesium (Cs)
Cesium is the most electropositive metal, meaning it readily loses its outer electron to form a positive ion (Cs⁺). This property makes cesium highly reactive, especially with water, where it reacts explosively, releasing hydrogen gas and heat. The electropositivity of cesium is valuable in scientific applications, including atomic clocks, where its precise frequency of electron transitions defines time measurement standards. In research, cesium is also used in photoelectric cells and in studies involving ionization energy and atomic structure. NEET students should recognize cesium’s role in demonstrating periodic trends in electropositivity and reactivity within the alkali metals group.
18. Liquid Non-metal: Bromine (Br)
Bromine is the only non-metal that exists as a liquid at room temperature, characterized by its dark reddish-brown color and a pungent odor. This halogen is highly reactive and is commonly used in various industries. In water treatment, bromine acts as a disinfectant, killing bacteria and other pathogens, while in fire retardants, it helps prevent the spread of flames. Bromine’s reactivity is due to its ability to form compounds easily, and its applications extend to pharmaceuticals, dyes, and photography. NEET aspirants should be familiar with bromine’s place in the halogen group elements, properties and its unique physical state, as these are often tested in chemistry exams.
19. Metal Kept in Paraffin Wax: Lithium (Li)
Lithium, an alkali metal, is stored under paraffin wax to prevent it from reacting with moisture and oxygen in the air. Lithium is highly reactive, even at room temperature, and exposure to air can cause it to oxidize rapidly, forming a dull surface coating. The wax acts as a barrier, maintaining lithium’s reactivity and purity. Lithium’s low atomic weight and high energy density make it a preferred material for rechargeable batteries, which are used in mobile phones, laptops, and electric vehicles. Lithium’s applications in energy storage technology make it one of the most important metals in modern society, a concept NEET students may encounter in questions on reactivity and storage of reactive metals.
20. Coinage Metals: Silver (Ag), Gold (Au), Copper (Cu), Aluminium (Al)
Historically, silver, gold, and copper have been used as coinage metals due to their resistance to corrosion, durability, and aesthetic appeal. These metals do not react easily with other substances, preserving their value and physical integrity over time. Silver and gold’s scarcity and appealing metallic luster have long made them symbols of wealth, while copper’s durability and ease of use make it suitable for everyday coins. Though aluminium is not traditionally a coinage metal, it is used in modern times due to its lightweight nature, corrosion resistance, and abundant availability. NEET aspirants should remember that coinage metals must be chemically stable and resistant to wear, qualities that make them ideal for minting currency.
Quick Recap Table – Properties of Elements
| No. | Property | Element/Ion |
|---|---|---|
| 1 | Smallest cation | H⁺ |
| 2 | Largest cation | Cs⁺ |
| 3 | Solid with highest density | Iridium (Ir) |
| 4 | Liquid with highest density | Mercury (Hg) |
| 5 | Elements named after countries | Ru, Ge, Po, Am |
| 6 | Most electronegative element | Fluorine (F) |
| 7 | Most abundant element on earth | Oxygen (O) |
| 8 | Most abundant metal in earth’s crust | Aluminium (Al) |
| 9 | Liquid metal | Mercury (Hg) |
| 10 | Lustrous non-metal | Iodine (I₂) |
| 11 | Hardest non-metal | Diamond |
| 12 | Soft metals | Na, K |
| 13 | Best ductile metals | Gold (Au), Silver (Ag) |
| 14 | Best conductor metal | Silver (Ag) |
| 15 | Most poisonous element | Plutonium (Pu) |
| 16 | Element with maximum isotopes | Silver (Ag) |
| 17 | Most electropositive metal | Cesium (Cs) |
| 18 | Liquid non-metal | Bromine (Br) |
| 19 | Metal kept in paraffin wax | Lithium (Li) |
| 20 | Coinage metals | Ag, Au, Cu, Al |
Conclusion
These elements and their unique properties highlight the diversity of the periodic table. NEET aspirants should focus on understanding how these traits affect an element’s behavior, applications, and role in different scientific contexts. Familiarity with such properties can give you an edge in exams by helping you quickly answer questions that test your foundational knowledge in chemistry.
To maximize your preparation:
- Connect the Dots: Link these properties to broader chemical concepts. For example, mercury’s liquid state at room temperature connects to concepts of metallic bonding and intermolecular forces.
- Practice Application: Use these properties to solve previous years’ NEET questions. Often, knowing that silver is the best conductor or fluorine is the most electronegative element can help you eliminate wrong options quickly.
- Visual Learning: Create your own mind maps connecting related elements and their properties. For instance, group all liquid elements together (mercury and bromine) or all reactive metals stored in special conditions.
- Regular Revision: Use the quick recap table as a daily revision tool, especially during the final weeks before NEET.
NEET aspirants can ensure a solid understanding of fundamental chemistry concepts by memorizing these properties and understanding associated facts about the elements.

